مهندسان جوش ( welding engineers )
جوشکاری یا عیب
مهندسان جوش ( welding engineers )
جوشکاری یا عیبجوشکاری و متالورژی جوشکاری
جوشکاری و متالورژی جوشکاری
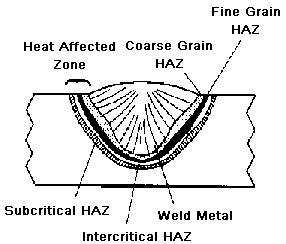
مقدمه ای بر جوشکاری و متالورژی جوشکاری : بیشتر سازه ها در صنعت از قطعات مختلف ( ریختگی ، آهنگری شده ، نوردی و ... ) تشکیل شده اند که با روش های گوناگونی بر یکدیگر متصل می شوند . روش های متفاوت اتصال فلزات به یکدیگر را برحسب نوع فرآیند و یا بنیان علمی آنها به دسته های مختلفی طبقه بندی نموده اند : الف ) روش های مکانیکی (پیچ ، پرچ ، پین ، خار و... ) ب) روش های متالورژیکی ( جوشکاری ، لحیم کاری و غیره ) ج) روش های شیمیائی ( چسب های معدنی و آلی ) و یا رده بندی بر اساس نوع اتصال : الف : روش های اتصال موقت ( پیچ و مهره ، پین و خار و....) ب : روش های اتصال نیمه موقت ( پرچ ، احتمالا لحیم کاری نرم و بعضی چسب ها ) ج : روش های اتصال دائم ( فرآیند جوشکاری و......) جوشکاری و رده بندی فرآیندهای جوشکاری : جوش ایده آل را می توان به محل اتصالی اطلاق نمود که نتوان آن موضع را از قسمت های دیگر قطعات جوش داده شده تشخیص داد .با وجود دست نیافتن به اینچنین مشخصات ، می توان خواص محل اتصال را چنان بالا برد که در عمل کاملا رضایتبخش باشد .نکته حائز اهمیت از نظر کارشناسی تشخیص نوع فلزی است که جوشکاری بر روی آن انجام می گیرد
ادامه مطلب ...انواع جوشکاری
. جوشکاری با قوس الکتریکی :
یکی از متداول ترین روشهای اتصال قطعات کار می باشد، ایجاد قوس الکتریکی عبارت از جریان مداوم الکترون بین دو الکترود و یا الکترود و یا الکترود و کار بوده که در نتیجه آن حرارت تولید می شود. باید توجه داشت که برای برقراری قوس الکتریک بین دو الکترود و یا کار و الکترود وجود هوا و یا یک گاز هادی ضروری است. بطوریکه در شرایط معمولی نمی توان در خلاء جوشکاری نمود.
در قوس الکتریکی گرما و انرژی نورانی در مکانهای مختلف یکسان نبوده بطوریکه تقریباً 43% از حرارت درآند و تقریباً 36% در کاتد و 21% بقیه بصورت قوس ظاهر می شود. دمای حاصله از قوس الکتریکی بنوع الکترودهای آن نیز وابسته است بطوریکه در قوس الکتریکی با الکترودهای ذغالی تا 3200 درجه سانتیگراد در کاتد و تا 3900 در آند حرارت وجود دارد. دمای حاصله در آندو کاتد برای الکترودهای فلزی حدوداً 2400 درجه سانتیگراد تا 2600 درجه تخمین زده شده است.
در این شرایط درجه حرارت در مرکز شعله بین 6000 تا 7000 درجه سانتیگراد می باشد از انرژی گرمائی حاصله در حالت فوق فقط 70% تا 60% در قوس الکتریک مشاهده گردیده که صرف ذوب کردن و عمل جوشکاری شده و بقیه آن یعنی 30% تا 40% بصورت تلفات گرمائی به محیط اطراف منتشر می گردد.
طول قوس شعله Arc length بین 8/0 تا 6/0 قطر الکترود می باشد و تقریباً 90% از قطرات مذاب جدا شده از الکترود به حوضچه مذاب وارد می گردد و 10% باطراف پراکنده می گردد. برای ایجاد قوس الکتریکی با ولتاژ کم بین 40 تا 50 ولت در جریان مستقیم و 60 تا 50 ولت در جریان متناوب احتیاج می باشد ولی در هر دو حالت شدت جریان باید بالا باشد نه ولتاژ.
ادامه مطلب ...انواع الکترودها
مشخصات الکترودها
انتخاب صحیح الکترود برای کار
انتخاب صحیح الکترود( از نظر قطر)
اطلاعات پاکت الکترود
تقسیم بندی الکترودها
الکترودهائی که در جوش اتصال فولاد به کار برده می شوند مفتولهای مغزی با آلیاژ یا بدون آلیاژ دارند که جریان جوش را هدایت می کند. شعله برق بین قطعه کار و سرآزاد الکترود می سوزد و الکترود به عنوان یک ماده اضافی ذوب می شود.
الکترودهای نرم شده دارای علائم اختصاری بوده ( دین 1913 ) که روی بسته بندی آنها نوشته شده است. علائم اختصاری تمام نکات مهمی که در به کار بردن آن الکترود باید مراعات شوند نشان می دهند.
ادامه مطلب ...
فر آیندهای جوشکاری با پرتو الکترونی

جوشکاری بروش الکترونی
بعد از اینکه در اواخر دهه 1950 جوشکاری EBW برای اولین بار به عنوان اولین پروسه جوشکاری استفاده شد، این فرآیند یک مقبولیت گسترده در صنعت بدست آورد. در ابتدا در صنایع هستهای بکار برده شد، و بعد مختصراً در صنایع فضایی و هواپیمایی، به کار گماشته شد، به سرعت تشخیص داده شد که فرآیند دارای ظرفیت لازم برای افزایش دادن کیفیت و قابلیت اعتماد قطعات بسیار حساس و بحرانی که در این صنایع استفاده شده است را دارد. فرآیند همچنین هزینه های ساخت را نیز کاهش داد. در طول این دورة ابتدایی کاربرد تجاری، فرآیند اکیداً برای عملیات در محفظة خلاء محدود شد. هر چند که سیستمی به زودی توسعه یافت که خلاء بالا تنها در قسمت تولید اشعه لازم بود. این سیستم اجازة انتخاب جوشکاری در یک محیط نیمه خلاء یا در یک محیط بدون خلاء را میدهد. این پیشرفت منجر بر مقبولیت آن توسط سازندگان خودروهای تجاری و سازندگان کالاهای معرفی شده در نتیجه جوشکاری EBW در رنج گستردهای از صنایع در کل جهان استفاده شده است. از اواخر دهة 1960 فرآیند هر دو تای جوش کم عمق و جوش بسیار عمیق تک پاسه را با کمترین مقدار اعوجاج حرارتی در قطعه کار را فراهم کرد.
ادامه مطلب ...جوشکاری پرتوالکترونی
فرایند جوشکاری پرتوالکترونی (EBW) فرایندی است که ذوب و اتصال فلزات در آن توسط حرارت حاصل از یک پرتوالکترونی صورت میگیرد. همان گونه که در شکل الف نشان داده شده است، کاتد تفنگ پرتوالکترونی، یک فیلمان (افروزه) با بار منفی است. هنگامی که این فیلمان تا بیش از دمای نشر گرمایونی آن گرم شود، از خود الکترون ساطع میکند. این الکترونها، توسط میدان الکتریکی بین الکترود شبکه با بار منفی (که کمی پایینتر از کاتد قرار گرفته است) و آند شتابدار میشوند. سپس از سوراخ روی آند عبور کرده و توسط یک سیمپیچ الکترومغناطیسی بر روی یک نقطه بر سطح قطعه کار متمرکز میشوند. جریان پرتو در روش EBW بین 50 تا A1000 و ولتاژ شتاب دهنده بین 30 تا KV175 متغیر است. یک پرتو الکترونی با شدت بسیار زیاد قادر است تا فلز را تبخیر کرده و یک سوراخ در حین جوشکاری در قطعه ایجاد کند که همان سوراخ کلیدی بوده و در شکل الف نشان داده شده است. شکل نشان میدهد که با کاهش فشار محیط، قطر پرتو کاهش مییابد. علت این امر آن است که الکترونها در اثر برخورد با مولکلوهای هوا پراکنده میشوند و بنابراین کاهش فشار محیط سبب کاهش پراکندگی آنها خواهد شد. این امر دلیل اصلی انجام فرایند EBW در محفظه خلاء میباشد.
ادامه مطلب ...آموزش نرم افزار Tekla-Xsteel
یک سری مثالهای آموزشی از نرم افزار Tekla-Xsteel براتون گذاشتم
رمز فایل : seismic.blogfa.com

معرفی دو نرم افزار آنلاین متالورژیکی
. نرم افزار آنلاین اول مربوط به رسم دیاگرام TTT و یا CCT فولاد می باشد. این دیاگرام ها در عملیات حرارتی فولاد بسیار مهم و کاربردی می باشند. برای دریافت دیاگرام برای فولاد مورد نظر ابتدا وارد نرم افزار آنلاین شوید:
فرم مربوطه را با توجه به مشخصات فولاد تکمیل کنید:
- درصد کربن، سیلیسیم، منگنز، نیکل، مولیبدن، کروم، وانادیم، کبالت فولاد را وارد کنید. مقدار بور را باید برحسب ppm (تعداد در ملیون) وارد کنید.
- دمای آستنیته فولاد را بر حسب کلوین وارد کنید، اگر می خواهید دمای Ae3 به عنوان دمای آستنیته فولاد در نظر گرفته شود عدد صفر را وارد کنید.
- حداقل و حداکثر سرعت سرد کردن را بر مبنای کلوین/ثانیه وارد کنید.
- در صورت نیاز می توانید حداقل و حداکثرزمان مندرج روی نمودار را تغییر دهید(بر حسب ثانیه).
-قسمت آخر مربوط به مشخصات استفاده کننده می باشد. نام، نام خانوداگی و آدرس ایمیل خود را وارد کنید.
برای مشاهده نمودار دکمه Make Graph را فشار دهید.
2. نرم افزار آنلاین دوم مربوط به پیش بینی ریزساختار و سختی ویکرز ناحیه جوش فولاد می باشد. برای به دست آوردن این مقادیر ابتدا وارد نرم افزار آنلاین شوید.
فرم مربوطه را با توجه به مشخصات فولاد تکمیل کنید، پر کردن فرم مشابه مورد قبل می باشد.
http://calculations.ewi.org/vjp/secure/AshbyModel.asp
انواع اتصال های جوشی
انواع اتصال های جوشی
1- اتصال لب به لب)Butt Joints ): برای اتصال ورق های مسطح با ضخامت های یکسان و یا تقریبا یکسان و همچنین جلوگیری از خروج از مرکزیت از این نوع درز جوش استفاده می شود. در این اتصالات معمولا از جوش شیاری با نفوذ کامل استفاده می شود.
2- اتصال رویهم (
جوش
اتصال قطعات فلزی به کمک حرارت به طوری که حرارت وارده آنها را به شکل خمیری و یا مذاب درآورد، فراین جوشکاری نامیده می شود.
انواع اتصال های جوشی
1- اتصال لب به لب)Butt Joints ): برای اتصال ورق های مسطح با ضخامت های یکسان و یا تقریبا یکسان و همچنین جلوگیری از خروج از مرکزیت از این نوع درز جوش استفاده می شود. در این اتصالات معمولا از جوش شیاری با نفوذ کامل استفاده می شود.
2- اتصال رویهم ( Lap Joints ): به دلیل سادگی اتصال دادن و سهولت در تنظیم اتصال به کار می رود. در این اتصالات اکثرا از جوش گوشه استفاده می شود.
3- اتصال گونیا ( Corner Joints ) : در این اتصالات از جوش گوشه استفاده می شود.
پیچیدگی یا اعوجاج (Distortion)
پیچیدگی یا اعوجاج (Distortion)
مقدمه
پیچیدگی و تغییر ابعاد یکی از مشکلاتی است که در اثر اشتباه طراحی و تکنیک عملیات جوشکاری ناشی میشود. با فرض اجتناب از ورود به مباحث تئوریک تنها به این مورد اشاره میکنیم که حین عملیات جوشکاری به دلیل عدم فرصت کافی برای توزیع یکنواخت بار حرارتی داده شده به موضع جوش و سرد شدن سریع محل جوش انقباضی که میبایست در تمام قطعه پخش میشد به ناچار در همان محدوده خلاصه میشود و این انقباض اگر در محلی باشد که از نظر هندسی قطعه زاویهدار باشد منجر به اعوجاج زاویهای (Angular distortion) میشود.
در نظر بگیرید تغییر زاویهای هرچند کوچک در قطعات بزرگ و طویل چه ایراد اساسی در قطعه نهایی ایجاد میکند. حال اگر خط جوش در راستای طولی و یا عرضی قطعه باشد اعوجاج طولی و عرضی (Longitudinal shrinkage or Transverse shrinkage) نمایان میشود. اعوجاج طولی و عرضی همان کاهش طول قطعه نهایی قطعه میباشد. این موارد هم بسیار حساس و مهم هستند. نوع دیگری از اعوجاج تاول زدن یا طبله کردن و یا قپه (Bowing) میباشد.
روشهای مقابله با اعوجاج
ادامه مطلب ...الکترودهای روپوش دار
آن چه مشخص است،هرچه پوشش الکترود ضخیم تر باشد ،جوش ازکیفیت بالاتری برخودار خواهدبود ،اما قیمت تمام شده تولیدآن نیز بیشتر خواهدشد.
جنس مفتول فلزی الکترود(مغزی الکترود)
باوجود آن که برای دستیابی به یک جوش مناسب،نزدیک بودن ترکیب شیمیایی الکترود به ترکیب شیمیایی فلز پایه از اهمیت ویژه ای برخورداراست،اما وجود پوشش های متنوع وفراوان،سبب شده تا سازندگان الکترود فقط از تعداد معدودی مغزی الکترود (باتنوع محدود ) برای تولید صدها نوع الکترود اقدام نمایند.
ادامه مطلب ...تحقیق WPS
مقدمه
هدف از تنظیم یک WPS مشخص و تعیین کردن جزئیات فرایند جوشکاری یک قطعه است برخی از کارخانه ها برای تولیدات خود گواهی کیفیت نیز تنظیم می کنند تا به وسیله آن شرایط آماده سازی بررسی و تائید مشخصات بیان شده در روش جوشکاری ،کنترل شود .
بر اساس نوع سازه ،استانداردهای مختلفی برای طراحی و ساخت سازه در کشورهای مختلف وجود است و تقریباً در تمامی این استانداردها بخشی به جوشکاری و کنترل کیفی اختصاص داده شده است. به عنوان مثال در آمریکا ،طراحی و ساخت بویلرها ف مخازن تحت فشار و نیرو گاه های اتمی بر اساس استانداردهای منتشره از سوی انجمن ASME صورت می گیرد....
ادامه مطلب ...لحیم کاری Soldering and Brazing
Soldering and Brazing
Soldering and Brazing are joining processes where parts are joined without melting the base metals. Soldering filler metals melt below 840 °F. Brazing filler metals melt above 840 °F. Soldering is commonly used for electrical connection or mechanical joints, but brazing is only used for mechanical joints due to the high temperatures involved.
Soldering and Brazing Benefits
|  |
Soldering and Brazing Issues
We can help optimize your joining process variables. Evaluate your current joining parameters and techniques. Help eliminate common joining problems and discontinuities such as those listed below:
Soldering and Brazing Joining Problems
|
|
If your company is experiencing these or other soldering or brazing problems you can engage AMC to improve your soldering or brazing processing. Hire our consultant to act as your soldering or brazing specialist
انتخاب و جوشکاری آلیاژها
Alloy Selection
Base metal and filler metal alloy selection is critical to producing good quality welds. Proper alloy selection can reduce numerous welding problems.
Does the aluminum welding you are performing result in significant reductions in tensile strength? Is the alloy you are using susceptible to cracking.? Does your weld need a post welding heat treatment? Are your processing parameters appropriate? Can your yield be improved? Can your weld quality be improved? Contact us about exotic alloys or common alloys as listed below:
Commonly used Welding Alloys
- Aluminum
- Steel
- Stainless Steel
- Titanium
- Nickel and Cobalt
- Magnesium
- Copper
Benefits from Proper Alloy Selection
- Increase weld quality and yield
- Proper weld joint strength
- Good corrosion and oxidation resistance
- Reduction of weld and HAZ cracking
- Eliminate reheat cracking
- Eliminate stress corrosion cracking
- Eliminate lamellar tearing
- Improved weldability
- Optimize dissimilar metal joints
Let AMC engineers help you identify proper base metal and filler rod alloys for your application. Select economical, good quality welding procedures to meet your customer needs. Let us optimize your welding procedure to improve yield, reduce rework, and reduce weld discontinuities.
Proper cleaning, processing, heat treating of your weld joint will reduce costs, reduce failures, and increase customer satisfaction. If your company is experiencing these or other welding problems you can retain AMC to improve your weld processing. Hire AMC to act as your welding specialist.
جوشکاری آلومینیوم و آلیاژهای آلومینیوم
Aluminum Welding
Aluminum is the most difficult alloy to weld. Aluminum oxide should be cleaned from the surface prior to welding. Aluminum comes in heat treatable and nonheat treatable alloys. Heat treatable aluminum alloys get their strength from a process called ageing. Significant decrease in tensile strength can occurs when welding aluminum due to over aging. For more information on aluminum welding processes, benefits of welding processes, welding discontinuities, or common welding problems please visit our homepage or any of the links to your left. Take advantage of our aluminum welding experience in developing your welding processes.
Welding Aluminum Alloys
Aluminum Alloys can be divided into nine groups.
Designation | Major Alloying Element |
1xxx | Unalloyed (pure) >99% Al |
2xxx | Copper is the principal alloying element, though other elements (Magnesium) may be specified |
3xxx | Manganese is the principal alloying element |
| 4xxx | Silicon is the principal alloying element |
| 5xxx | Magnesium is the principal alloying element |
| 6xxx | Magnesium and Silicon are principal alloying elements |
| 7xxx | Zinc is the principal alloying element, but other elements such as Copper, Magnesium, Chromium, and Zirconium may be specified |
| 8xxx | Other elements (including Tin and some Lithium compositions) |
9xxx | Reserved for future use |
Aluminum alloys are readily available in various product forms. To establish a proper welding procedure it is necessary to know the material properties of the Aluminum alloy being welded.
Below are some of the factors affecting the welding of Aluminum.
Aluminum Oxide Coating
Thermal Conductivity
Thermal Expansion Coefficient
Melting Characteristics
Wrought Aluminum Alloys
1xxx Series. These grades of aluminum are characterized by excellent corrosion resistance, high thermal and electrical conductivities, low mechanical properties, and excellent workability. Moderate increases in strength may be obtained by strain hardening. Iron and silicon are the major impurities.
2xxx Series. These alloys require solution heat treatment to obtain optimum properties; in the solution heat-treated condition, mechanical properties are similar to, and sometimes exceed, those of low-carbon steel. In some instances, precipitation heat treatment (aging) is employed to further increase mechanical properties. This treatment increases yield strength, with attendant loss in elongation; its effect on tensile strength is not as great.
The alloys in the 2xxx series do not have as good corrosion resistance as most other aluminum alloys, and under certain conditions they may be subject to intergranular corrosion. Alloys in the 2xxx series are good when some strength at moderate temperatures is desired. These alloys have limited weldability, but some alloys in this series have superior machinability.
3xxx Series. These alloys generally are non-heat treatable but have about 20% more strength than 1xxx series alloys. Because only a limited percentage of manganese (up to about 1.5%) can be effectively added to aluminum, manganese is used as a major element in only a few alloys.
4xxx Series. The major alloying element in 4xxx series alloys is silicon, which can be added in sufficient quantities (up to 12%) to cause substantial lowering of the melting range. For this reason, aluminum-silicon alloys are used in welding wire and as brazing alloys for joining aluminum, where a lower melting range than that of the base metal is required. The alloys containing appreciable amounts of silicon become dark gray to charcoal when anodic oxide finishes are applied and hence are in demand for architectural applications.
5xxx Series. The major alloying element is Magnesium and when it is used as a major alloying element or with manganese, the result is a moderate-to-high-strength work-hardenable alloy. Magnesium is considerably more effective than manganese as a hardener, about 0.8% Mg being equal to 1.25% Mn, and it can be added in considerably higher quantities. Alloys in this series possess relatively good welding characteristics and relatively good resistance to corrosion in marine atmospheres. However, limitations should be placed on the amount of cold work and the operating temperatures permissible for the higher-magnesium alloys to avoid susceptibility to stress-corrosion cracking.
6xxx Series. Alloys in the 6xxx series contain silicon and magnesium approximately in the proportions required for formation of magnesium silicide (Mg2Si), thus making them heat treatable. Although not as strong as most 2xxx and 7xxx alloys, 6xxx series alloys have relatively good formability, weldability, machinability, and relatively good corrosion resistance, with medium strength. Alloys in this heat-treatable group are sometimes formed in the T4 temper (solution heat treated but not precipitation heat treated) and strengthened after forming to full T6 properties by precipitation heat treatment.
7xxx Series. Zinc, in amounts of 1 to 8% is the major alloying element in 7xxx series alloys, and when coupled with a smaller percentage of magnesium results in heat-treatable alloys of moderate to high strength. Usually other elements, such as copper and chromium, are also added in small quantities. Some 7xxx series alloys have been used in airframe structures, and other highly stressed parts. Higher strength 7xxx alloys exhibit reduced resistance to stress corrosion cracking and are often utilized in an overaged temper to provide better combinations of strength, corrosion resistance, and fracture toughness.
Aluminum Welding Services
Visit our homepage for detailed information on arc welding processes, welding procedures, weld failure analysis, and expert witness testimony. AMC can solve your companies aluminum welding procedure problems. Hire AMC to act as your welding specialist.
جوشکاری فولادهای زنگ نزن Welding Stainless Steel
Welding Stainless Steel
The stainless properties of stainless steels are primarily due to the presence of chromium in quantities greater than roughly 12 weight percent. This level of chromium is the minimum level of chromium to ensure a continuous stable layer of protective chromium-rich oxide forms on the surface. The ability to form chromium oxide in the weld region must be maintained to ensure stainless properties of the weld region after welding. In commercial practice, however, some stainless steels are sold containing as little as 9 weight percent chromium and will rust at ambient temperatures.
Stainless steels are generally classified by their microstructure and are identified as ferritic, martensitic, austenitic, or duplex (austenitic and ferritic). The microstructure significantly affects the weld properties and the choice of welding procedure used for these stainless steel alloys. In addition, a number of precipitation-hardenable (PH) stainless steels exist. Precipitation-hardenable stainless steels have martensitic or austenitic microstructures.
Iron, carbon, chromium and nickel are the primary elements found in stainless steels and significantly affect microstructure and welding. Other alloying elements are added to control microstructure or enhance material properties. These other alloys affect welding properties by changing the chromium or nickel equivalents and thereby changing the microstructure of the weld metal. Generally, 200 and 300 series alloys are mostly austenitic and 400 series alloys are ferritic or martensitic, but exceptions exist.
Stainless steels are subject to several forms of localized corrosive attack. The prevention of localized corrosive attack is one of the concerns when selecting base metal, filler metal and welding procedures when fabricating components from stainless steels.
Stainless steels are subject to weld metal and heat affected zone cracking, the formation of embrittling second phases and concerns about ductile to brittle fracture transition. The prevention of cracking or the formation of embrittling microstructures is another main concern when welding or fabricating stainless steels.
Welding Austenitic Stainless Steels
Ideally, austenitic stainless steels exhibit a single-phase, the face-centered cubic (fcc) structure, that is maintained over a wide range of temperatures. This structure results from a balance of alloying additions, primarily nickel, that stabilize the austenite phase from elevated to cryogenic temperatures. Because these alloys are predominantly single phase, they can only be strengthened by solid-solution alloying or by work hardening. Precipitation-strengthened austenitic stainless steels will be discussed separately below.
The austenitic stainless steels were developed for use in both mild and severe corrosive conditions. Austenitic stainless steels are used at temperatures that range from cryogenic temperatures, where they exhibit high toughness, to elevated temperatures, where they exhibit good oxidation resistance. Because the austenitic materials are nonmagnetic, they are sometimes used in applications where magnetic materials are not acceptable.
The most common types of austenitic stainless steels are the 200 and 300 series. Within these two grades, the alloying additions vary significantly. Furthermore, alloying additions and specific alloy composition can have a major effect on weldability and the as-welded microstructure. The 300 series of alloys typically contain from 8 to 20 weight percent Ni and from 16 to 25 weight percent Cr.
A concern, when welding the austenitic stainless steels, is the susceptibility to solidification and liquation cracking. Cracks can occur in various regions of the weld with different orientations, such as centerline cracks, transverse cracks, and microcracks in the underlying weld metal or adjacent heat-affected zone (HAZ). These cracks are primarily due, to low-melting liquid phases, which allow boundaries to separate under the thermal and shrinkage stresses during weld solidification and cooling.
Even with these cracking concerns, the austenitic stainless steels are generally considered the most weldable of the stainless steels. Because of their physical properties, the welding behavior of austenitic stainless steels is different than the ferritic, martensitic, and duplex stainless steels. For example, the thermal conductivity of austenitic alloys is roughly half that of ferritic alloys. Therefore, the weld heat input that is required to achieve the same penetration is reduced. In contrast, the coefficient of thermal expansion of austenite is 30 to 40 percent greater than that of ferrite, which can result in increases in both distortion and residual stresses, due to welding. The molten weld pool of the austenitic stainless steels is commonly more viscous, or sluggish, than ferritic and martensitic alloys. This slows down the metal flow and wettability of welds in austenitic alloys, which may promote lack-of-fusion defects when poor welding procedures are employed.
Welding Ferritic Stainless Steels
Ferritic stainless steels comprise approximately half of the 400 series stainless steels. These steels contain from 10.5 to 30 weight percent chromium along with other alloying elements, particularly molybdenum. Ferritic stainless steels are noted for their stress-corrosion cracking (SCC) resistance and good resistance to pitting and crevice corrosion in chloride environments, but have poor toughness, especially in the welded condition.
Ideally, ferritic stainless steels have the body-centered cubic (bcc) crystal structure known as ferrite at all temperatures below their melting temperatures. Many of these alloys are subject to the precipitation of undesirable intermetallic phases when exposed to certain temperature ranges. The higher-chromium alloys can be embrittled by precipitation of the tetragonal sigma phase, which is based on the compound FeCr.
Molybdenum promotes formation of the complex cubic chi phase, which has a nominal composition of Fe36Cr12Mo10. Embrittlement increases with increasing chromium plus molybdenum contents. It is generally agreed that the severe embrittlement which occurs upon long-term exposure is due to the decomposition of the iron-chromium ferrite phase into a mixture of iron-rich alpha and chromium-rich alpha-prime phases. This embrittlement is often called "alpha-prime embrittlement." Additional reactions such as chromium carbide and nitride precipitation may play a significant role in the more rapid, early stage 885 °F embrittlement.
The ferritic stainless steels have higher yield strengths and lower ductilities than austenitic stainless steels. Like carbon steels, and unlike austenitic stainless steels, the ferritic stainless alloys exhibit a transition from ductile-to-brittle behavior as the temperature is reduced, especially in notched impact tests. The ductile-to-brittle transition temperature (DBTT) for the ultrahigh-purity ferritic stainless steels is lower than that for standard ferritic stainless steels. It is typically below room temperature for the ultrahigh-purity ferritic stainless steels. Nickel additions lower the DBTT and there by slightly increase the thicknesses associated with high toughness. Nevertheless, with or without nickel, the ferritic stainless steels would need engineering review for anything other than thin walled applications as they are prone to brittle failure.
Welding Martensitic Stainless Steels
Martensitic stainless steels are considered to be the most difficult of the stainless steel alloys to weld. Higher carbon contents will produce greater hardness and, therefore, an increased susceptibility to cracking.
In addition to the problems that result from localized stresses associated with the volume change upon martensitic transformation, the risk of cracking will increase when hydrogen from various sources is present in the weld metal. A complete and appropriate welding process is needed to prevent cracking and produce a sound weld.
Martensitic stainless steels are essentially alloys of chromium and carbon that possess a body-centered cubic (bcc) or body-centered tetragonal (bct) crystal structure (martensitic) in the hardened condition. They are ferromagnetic and hardenable by heat treatments. Their general resistance to corrosion is adequate for some corrosive environments, but not as good as other stainless steels.
The chromium content of these materials generally ranges from 11.5 to 18 weight percent, and their carbon content can be as high as 1.2 weight percent. The chromium and carbon contents are balanced to ensure a martensitic structure after hardening. Martensitic stainless steels are chosen for their good tensile strength, creep, and fatigue strength properties, in combination with moderate corrosion resistance and heat resistance.
The most commonly used alloy within this stainless steel family is type 410, which contains about 12 weight percent chromium and 0.1 weight percent carbon to provide strength. Molybdenum can be added to improve mechanical properties or corrosion resistance. Nickel can be added for the same reasons. When higher chromium levels are used to improve corrosion resistance, nickel also serves to maintain the desired microstructure and to prevent excessive free ferrite. The limitations on the alloy content required to maintain the desired fully martensitic structure restrict the obtainable corrosion resistance to moderate levels.
Welding Duplex Stainless Steels
Duplex stainless steels are two phase alloys based on the iron-chromium-nickel system. Duplex stainless steels usually comprise approximately equal proportions of the body-centered cubic (bcc) ferrite and face-centered cubic (fcc) austenite phases in their microstructure and generally have a low carbon content as well as, additions of molybdenum, nitrogen, tungsten, and copper. Typical chromium contents are 20 to 30 weight percent and nickel contents are 5 to 10 weight percent. The specific advantages offered by duplex stainless steels over conventional 300 series stainless steels are strength, chloride stress-corrosion cracking resistance, and pitting corrosion resistance.
Duplex stainless steels are used in the intermediate temperature ranges from ambient to several hundred degrees Fahrenheit (depending on environment), where resistance to acids and aqueous chlorides is required. The weldability and welding characteristics of duplex stainless steels are better than those of ferritic stainless steels, but generally not as good as austenitic materials.
A suitable welding process is needed to obtain sound welds. Duplex stainless steel weldability is generally good, although it is not as forgiving as austenitic stainless steels. Control of heat input is important. Solidification cracking and hydrogen cracking are concerns when welding duplex stainless steels, but not as significant for some other stainless steel alloys.
Current commercial grades of duplex stainless steels contain between 22 and 26 weight percent chromium, 4 to 7 weight percent nickel, up to 4.5 weight percent molybdenum, as well as some copper, tungsten, and nitrogen. Modifications to the alloy compositions have been made to improve corrosion resistance, workability, and weldability. In particular, nitrogen additions have been effective in improving pitting corrosion resistance and weldability.
The properties of duplex stainless steels can be appreciably affected by welding. Due to the importance of maintaining a balanced microstructure and avoiding the formation of undesirable metallurgical phases, the welding procedures must be properly specified and controlled. If the welding procedure is improper and disrupts the appropriate microstructure, loss of material properties can occur.
Because these steels derive properties from both austenitic and ferritic portions of the structure, many of the single-phase base material characteristics are also evident in duplex materials. Austenitic stainless steels have good weldability and low-temperature toughness, whereas their chloride SCC resistance and strength are comparatively poor. Ferritic stainless steels have good resistance to chloride SCC but have poor toughness, especially in the welded condition. A duplex microstructure with high ferrite content can therefore have poor low-temperature notch toughness, whereas a structure with high austenite content can possess low strength and reduced resistance to chloride SCC.
The high alloy content of duplex stainless steels also makes them susceptible to the formation of intermetallic phases from extended exposure to high temperatures. Significant intermetallic precipitation may lead to a loss of corrosion resistance and sometimes to a loss of toughness.
Duplex stainless steels have roughly equal proportions of austenite and ferrite, with ferrite being the matrix. The duplex stainless steels alloying additions are either austenite or ferrite formers. This is occurs by extending the temperature range over which the phase is stable. Among the major alloying elements in duplex stainless steels chromium and molybdenum are ferrite formers, whereas nickel, carbon, nitrogen, and copper are austenite formers.
Composition also plays a major role in the corrosion resistance of duplex stainless steels. Pitting corrosion resistance can be adversely affected. To determine the extent of pitting corrosion resistance offered by the material, a pitting resistance equivalent is commonly used.
Welding Precipitation-Hardenable Stainless Steels
Precipitation-hardening (PH) stainless steels are iron-chromium-nickel alloys. They generally have better corrosion resistance than martensitic stainless steels. The high tensile strengths of the PH stainless steels is due to precipitation hardening of a martensitic or austenitic matrix. Copper, aluminum, titanium, niobium (columbium), and molybdenum are the primary elements added to these stainless steels to promote precipitation hardening.
Precipitation-hardening stainless steels are commonly categorized into three types martensitic, semiaustenitic, and austenitic based on their martensite start and finish (Ms and Mf) temperatures and the resulting microstructures. The issues involved in welding PH steels are different for each group.
It is important to understand the microstructure of the particular type of alloy being welded. Some of the PH stainless steels solidify as primary ferrite and have relatively good resistance to hot cracking. In other PH stainless steels, ferrite is not formed, and it is more difficult to weld these alloys without hot cracking.
If your company is experiencing these or other welding problems you can retain AMC to improve your weld processing. Hire AMC to act as your welding specialist.
Welding Steel Alloys
Welding Steel Alloys
Steel Alloys can be divided into five groups
Steels are readily available in various product forms. To establish a proper welding procedure it is necessary to know the material properties of the steel being welded. The American Iron and Steel Institute defines carbon steel as follows: |
Steel is considered to be carbon steel when no minimum content is specified or required for chromium, cobalt, columbium [niobium], molybdenum, nickel, titanium, tungsten, vanadium or zirconium, or any other element to be added to obtain a desired alloying effect; when the specified minimum for copper does not exceed 0.40 per cent; or when the maximum content specified for any of the following elements does not exceed the percentages noted: manganese 1.65, silicon 0.60, copper 0.60. Carbon steels are normally classified as shown below.
Low-carbon steels contain up to 0.30 weight percent C. The largest category of this class of steel is flat-rolled products (sheet or strip) usually in the cold-rolled and annealed condition. The carbon content for these high-formability steels is very low, less than 0.10 weight percent C, with up to 0.4 weight percent Mn. For rolled steel structural plates and sections, the carbon content may be increased to approximately 0.30 weight percent, with higher manganese up to 1.5 weight percent.
Medium-carbon steels are similar to low-carbon steels except that the carbon ranges from 0.30 to 0.60 weight percent and the manganese from 0.60 to 1.65 weight percent. Increasing the carbon content to approximately 0.5 weight percent with an accompanying increase in manganese allows medium-carbon steels to be used in the quenched and tempered condition.
High-carbon steels contain from 0.60 to 1.00 weight percent C with manganese contents ranging from 0.30 to 0.90 weight percent.
High-strength low-alloy (HSLA) steels, or microalloyed steels, are designed to provide better mechanical properties than conventional carbon steels. They are designed to meet specific mechanical properties rather than a chemical composition. The chemical composition of a specific HSLA steel may vary for different product thickness to meet mechanical property requirements. The HSLA steels have low carbon contents (0.50 to ~0.25 weight percent C) in order to produce adequate formability and weldability, and they have manganese contents up to 2.0 weight percent. Small quantities of chromium, nickel, molybdenum, copper, nitrogen, vanadium, niobium, titanium, and zirconium are used in various combinations.
Below are some typical welding considerations when welding carbon and low alloy steels
- Carbon Equivalent of the Steel
- Weld Cooling Rates
- Solidification Cracking
- Reheat Cracking
- Lamellar Tearing
- Hydrogen Cracking
If your company is experiencing these or other welding problems you can retain AMC to improve your weld processing. Hire AMC to act as your welding specialist.
DISTORTION
DISTORTION
Welding involves highly localized heating of the metal being joined together. The temperature distribution in the weldment is therefore nonuniform. Normally, the weld metal and the heat affected zone (HAZ) are at temperatures substantially above that of the unaffected base metal. Upon cooling, the weld pool solidifies and shrinks, exerting stresses on the surrounding weld metal and HAZ.
If the stresses produced from thermal expansion and contraction exceed the yield strength of the parent metal, localized plastic deformation of the metal occurs. Plastic deformation results in lasting change in the component dimensions and distorts the structure. This causes distortion of weldments.
Several types of distortion are listed below:
- Longitudinal shrinkage
- Transverse shrinkage
- Angular distortion
- Bowing
- Buckling
- Twisting
Factors affecting distortion
If a component were uniformly heated and cooled distortion would be minimized. However, welding locally heats a component and the adjacent cold metal restrains the heated material. This generates stresses greater than yield stress causing permanent distortion of the component. Some of the factors affecting the distortion are listed below:
- Amount of restraint
- Welding procedure
- Parent metal properties
- Weld joint design
- Part fit up
Restraint can be used to minimize distortion. Components welded without any external restraint are free to move or distort in response to stresses from welding. It is not unusual for many shops to clamp or restrain components to be welded in some manner to prevent movement and distortion. This restraint does result in higher residual stresses in the components.
Welding procedure impacts the amount of distortion primarily due to the amount of the heat input produced. The welder has little control on the heat input specified in a welding procedure. This does not prevent the welder from trying to minimize distortion. While the welder needs to provide adequate weld metal, the welder should not needlessly increase the total weld metal volume added to a weldment.
Parent metal properties, which have an effect on distortion, are coefficient of thermal expansion and specific heat of the material. The coefficient of thermal expansion of the metal affects the degree of thermal expansion and contraction and the associated stresses that result from the welding process. This in turn determines the amount of distortion in a component.
Weld joint design will effect the amount of distortion in a weldment. Both butt and fillet joints may experience distortion. However, distortion is easier to minimize in butt joints.
Part fit up should be consistent to fabricate foreseeable and uniform shrinkage. Weld joints should be adequately and consistently tacked to minimize movement between the parts being joined by welding.
If your company is experiencing these or other welding problems you can engage AMC to reduce your welding distortion. Hire AMC to act as your welding specialist.
جوشکاری سازه آیین نامه ها Structural Welding Codes
سازه آیین نامه ها چندین سازه جوش آیین نامه ها وجود دارد. این صفحه وب را فراهم رئوس مطالب جوشکاری سازه کود. مثالهای نمونه از این زیر لیست شده اند عبارتند از :
فولاد (AWS D1.1)
آلومینیوم (AWS D1.2)
آرماتور فلزی (AWS D1.4)
فولاد ضد زنگ (AWS D1.6)
بررسی اجمالی
کدهای جوشکاری سازه پوشش جنبه های مختلف برای ساخت و احداث سازه های جوش داده شده. در حالی که از تغییرات در قوانین جزایی را از زمان به زمان ارائه میدهد که در اینجا ارائه یک مرور کلی از اطلاعات موجود در کد وجود دارد.
برای مقایسه کدهای جوش سازه جامع تر از بخش نهم تحت فشار بویلر و مخازن ASME کد (BPVC) ، از مسائل از قبیل طراحی و ساخت هستند ، در بخش دیگری از ASME BPVC خطاب. برخی از نمونه های مورد نیاز جوشکاری خطاب توسط آیین نامه ها سازه جوش عبارتند از :
طراحی اتصالات جوش داده شده
الزامات مورد نیاز برای جوش مشخصات روش کار (WPS)
پرسنل مورد نیاز برای جوشکاری عملکرد تحصیلی مدرک
ساخت مورد نیاز
بازرسی
طراحی جوش
مهندسان به طور معمول اتصالات جوش داده شده و مطابق با الزامات آن شناسایی شده در جوشکاری کد طرح. کد آدرس جنبه های مختلف جوشکاری. برخی از مسائل جوشکاری زیر مشخص شده اند
welds شیاردار
welds گچ بری
طول جوش
کامل نفوذ مشترک
نفوذ ناقص مشترک
شلپ شلپ کردن مفاصل
اندازه جوش
فاصله جوش
انتقال قدرت
سازههای ثابت و ادواری بارگذاری
مدرک
مشخصات روش جوشکاری (WPS) و عملکرد تحصیلی مدرک جوش پرسنل مورد نیاز است. مدرک تحصیلی را پوشش میدهد جنبههای مختلف مربوط به تولید welds. برخی از این آیتم ها در زیر آورده شده عبارتند از :
فرآیند جوش (SMAW ، GMAW ، FCAW ، GTAW ، ص و غیره)
فلز پایه
فلز پر کننده
قبلا گرم و درجه حرارت Interpass
شدت جریان برق
ولتاژ
سرعت سفر
گاز محافظ
ضخامت
موقعیت جوش
پشتیبانی
مشخصات مورد نیاز برای روش جوشکاری و جوشکاری پرسنل عملکرد تحصیلی مدرک در کدهای شناسایی می شوند.
ساخت
ساخت و نصب قطعات و سازه های جوش داده شده در کد شده اند. برخی از اقلام تحت پوشش کد زیر فهرست شده اند
فلز پایه
مواد مصرفی جوشکاری
قبلا گرم و درجه حرارت Interpass
تسکین استرس حرارتی
پشتوانه ، پشتوانه گاز ، یا درج
محیط زیست جوش
تطابق طراحی
آمادگی و پایه فلزی
ابعاد و تلرانس
مشخصات جوش
تعمیرات
بازرسی
مدارک مورد نیاز برای بازرس و مسئولیت ، معیارهای پذیرش برای discontinuities و روشهایی برای آزمایش غیرمخرب (غیر مخرب) را در کد شناسایی می شوند. برخی از موارد شناسایی شده در کد زیر آمده است :
بازرس
مواد
WPS
تجهیزات
جوشکار مدرک
Welds
سوابق
ضوابط پذیرش
البدل ضوابط پذیرش
تست مایع نافذ
تست ذرات مغناطیسی
رادیوگرافی بازرسی
التراسونیک بازرسی
اگر شرکت شما است با این مسائل را تجربه و یا مشکلات جوشکاری دیگر شما می توانید AMC پردازش جوش خود را بهبود بخشید را حفظ کنید. استخدام مشاور ما در عمل به عنوان متخصص جوش دهید
Structural Welding Codes
There are several Structural Welding Codes. This web page provides an outline of the Structural Welding Codes. Typical examples of these are listed below:
Steel (AWS D1.1)
Aluminum (AWS D1.2)
Reinforcing Steel (AWS D1.4)
Stainless Steel (AWS D1.6)
Overview
The Structural welding Codes cover various aspects for fabricating and erecting welded structures. While there are changes to the Codes from time to time the outlines here provide an overview of the information in the codes.
For comparison the Structural Welding Codes are more comprehensive than Section IX of the ASME Boiler and Pressure Vessel Code (BPVC), as issues such as design and fabrication are addressed in other sections of the ASME BPVC. Some examples of welding requirements addressed by the Structural Welding Codes include:
Design of welded connections
Requirements for Welding Procedure Specifications (WPS)
Requirements for Welding Personnel Performance Qualification
Fabrication Requirements
Inspection
Weld Design
Engineers typically design welded connections in accordance with the requirements identified in the welding code. The codes address various aspects of the weld. Some of the weld issues specified are listed below
Groove welds
Fillet welds
Weld length
Complete joint penetration
Partial joint penetration
Lap joints
Weld size
Weld spacing
Transitions
Static and cyclical loading
Qualification
Welding Procedure Specifications (WPS) and Welding Personnel Performance Qualification are required. The qualification covers various aspects concerning the production of welds. Some of these items are listed below:
Welding Process (SMAW, GMAW, FCAW, GTAW, SAW etc.)
Base metal
Filler metal
Preheat and Interpass temperature
Amperage
Voltage
Travel speed
Shielding gas
Thickness
Backing
Requirements for Welding procedure Specifications and Welding Personnel Performance Qualification are identified in the Codes.
Fabrication
Fabrication and erection of welded assemblies and structures are detailed in the code. Some of the items covered by the code are listed below
Base metal
Welding consumables
Preheat and Interpass temperature
Stress relief heat treatment
Backing, backing gas, or inserts
Welding environment
Design compliance
Preparation of base metal
Dimensions and Tolerances
Weld profile
Repairs
Inspection
Requirements for the Inspector's qualifications and responsibilities, acceptance criteria for discontinuities, and procedures for nondestructive testing (NDT) are identified in the Code. Some of the items identified in the code are listed below:
Inspector
Materials
WPS
Equipment
Welder Qualification
Welds
Records
Acceptance Criteria
Alternate Acceptance Criteria
Liquid Penetrant Testing
Magnetic Particle Testing
Radiographic Inspection
Ultrasonic Inspection
If your company is experiencing issues with these or other welding problems you can retain AMC to improve your weld processing. Hire our consultant to act as your welding specialist.
Welding Discontinuities
Welding Discontinuities
Some examples of welding discontinuities are shown below. Evaluation of the discontinuity will determine if the discontinuity is a defect or an acceptable condition:
| Incomplete Fusion - A weld discontinuity in which fusion did not occur between weld metal and fusion faces or adjoining weld beads. | |
| Undercut - A groove melted into the base metal adjacent to the weld toe or weld root and left unfilled by weld metal. Overlap - The protrusion of weld metal beyond the weld toe or weld root. | |
| Underfill - A condition in which the weld face or root surface extends below the adjacent surface of the base metal. Incomplete Joint Penetration - A joint root condition in a groove weld in which weld metal does not extend through the joint thickness | |
| Partial joint penetration groove welds are commonly specified in lowly loaded structures. However, incomplete joint penetration when a full penetration joint is required, as depicted above, would be cause for rejection. A fix for an incomplete penetration joint would be to back gouge and weld from the other side. Another acceptable partial penetration joint is shown below. | |
| Partial penetration joint on the left without discontinuities is an acceptable condition where appropriate. Appropriate engineering decisions need to be applied to determine what type of joint should be specified for a given application. | |
Engineering should be contacted to determine whether partial penetration of full penetration joints are appropriate for a particular situation.
Above are several different representations of weld Cracking
Below is a representation of a convex fillet weld without discontinuities.
If your company is experiencing these or other welding problems you can retain AMC to improve your weld processing. Hire AMC to act as your welding specialist.